Subscribe to our newsletter and receive exclusive offers every week
Thank you for Subscribe.


Highlighting the complexity of diagnosing infectious diseases in clinical settings, the review underscores the limitations of traditional methods such as microbiological culture and multiplex PCR tests. It introduces mcfDNA sequencing as a promising technology for diagnosing infectious etiologies, currently available in select reference laboratories.
Clinical Applications and Availability:
This section delves into the commercial availability of mcfDNA sequencing tests, with a focus on prominent examples like the Karius test. It outlines the diverse applications of mcfDNA sequencing, including its role in diagnosing invasive fungal diseases, community-acquired pneumonia, and infections in immunocompromised hosts.
Non-Invasive Testing Landscape:
The review explores the non-invasive nature of liquid biopsy tests based on circulating cell-free DNA (cfDNA). It elaborates on the origins of cfDNA in circulation and its role as a biomarker in clinical practice, particularly in prenatal screening, transplantation, and oncology.
Early Detection Methods:
Detailing the arsenal of early detection methods for mcfDNA, the review emphasizes the significance of various PCR techniques. It elucidates how mcfDNA-based next-generation sequencing (mcfDNA sequencing) serves as a hypothesis-free test, shedding light on its potential to identify a wide range of infections in a single run.
mcfDNA Sequencing Process:
Figure 1B outlines the principle and procedure of the mcfDNA sequencing test for identifying pathogens causing invasive infection. The review categorizes the mcfDNA sequencing process into pre-analytical, analytical, and post-analytical phases, providing a structured approach for clinicians and researchers.
Biological Characteristics of mcfDNA:
This section explores the biological characteristics of mcfDNA, discussing its concentration in healthy individuals and variations in pathological conditions. Figure 1A depicts the possible sources of mcfDNA in circulation. The mcfDNA sequencing process can be divided into pre-analytical, analytical, and post-analytical phases (Figure 1B).
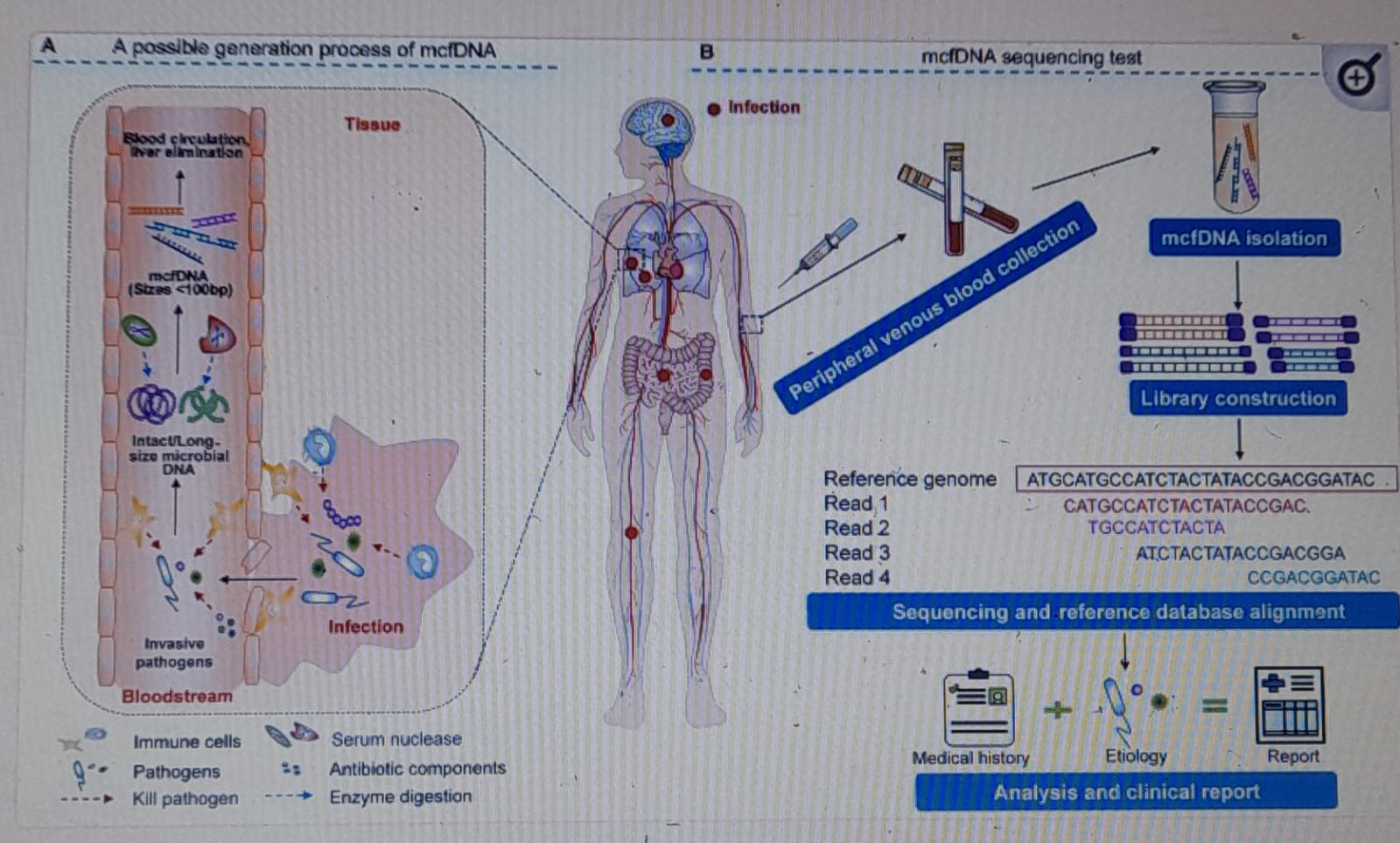
Figure1: The principle and procedure of the mcfDNA sequencing test for identifying pathogens causing invasive infection (Aadpted from, Dongsheng et al., Theranostics 2020, Vol. 10, Issue 12).
Conclusion:
The review concludes by underscoring the growing interest in clinical metagenomic cell-free DNA sequencing in pathology labs. It points to the traction gained by the mcfDNA sequencing test for infection diagnosis, highlighting its potential for faster actionable diagnoses compared to conventional microbiologic methods. The urgent need for research, particularly multicenter prospective cohort studies in Indian patients, is emphasized to determine the broader impact of mcfDNA sequencing in routine clinical diagnosis.
This comprehensive review serves as a valuable resource, offering practitioners and researchers insights into the evolving landscape of microbial cell-free DNA sequencing and its potential applications in clinical metagenomics.
Author:
Dr. K.M. Singh, Head of Molecular Diagnostics at Sterling Accuris, leverages 20+ years of expertise, spearheading advancements on Sanger Sequencing, and NGS (Next Generation Sequencing).
Featured Health Blogs